It often happens when you do not water a plant for a few days and they become dry and a little lifeless. In the language of science, what happens is the water moves out of the cells, leaving it dry and lifeless. The process that happens is what we call Osmosis.
In the above case, what happens is due to the absence of water in the extracellular space; the water moves out.
Osmosis is a process in which there is a movement of molecules that are solvent. These molecules move through a permeable membrane. This movement happens from a high water potential in a specific direction. In this direction, the solute concentrations get equalized.
To know more about Osmosis, its types, importance, and examples, keep reading. We are going to let you know all the information that you know about Osmosis.
What Is Osmosis, And How Does It Happen?
Osmosis happens when solvent moves across a semipermeable membrane towards a solute. This solute is of higher concentration. In biology, the solvent is in general water, but Osmosis can happen in other liquids too. It can happen in supercritical liquids and also in gases.
To understand the concept of Osmosis, let’s take the example of water as a solute. Now, as per the Osmosis process, the water molecules will pass from the cell membrane. The molecules will move from the area of low concentration to the area of high concentration.
If the cells get submerged in saltwater, then the water molecules move out from the cell. And if the cell gets submerged in fresh water, then the water molecules move inside the cell.
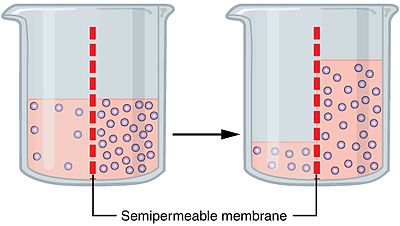
Osmotic Solution
In general, there are three forms of Osmotic Solution. These three types are as follows:
- Hypertonic Solution
- Isotonic Solution
- Hypotonic Solution
Hypertonic Solution
To understand most simply, in a hypertonic solution, water leaves the cell. Hence leaving the cells to shrink.
Isotonic Solution
Now in the Isotonic solution, what happens is there is no water movement. Hence you will find that there is no change in the size of the cell.
Hypotonic Solution
Whereas what happens in a Hypotonic Solution is very different from the rest of the two. In a hypotonic solution, the water in fact moves inside of the cell. This causes the cells to swell.
Tonicity is an important aspect of all living organisms. The reason being they often lack rigid cell walls. And the living environment is either hypertonic or hypotonic.
Now that we have learned about the Osmotic solutions let us peek at the type of Osmosis.
Types of Osmosis
Osmosis happens in two major types. The types of Osmosis are as follows:
Endosmosis
If a cell gets placed in a hypotonic solution, the result is that the water moves inside of the cell. Thus, the cell gets swelled or, in other words, gets deplasmolysed.
Exosmosis
On the other side, if the cell gets placed in a hypertonic solution, the water moves out of the cell. The result being, the cells become flaccid. Or gets plasmolyzed.
Osmotic Pressure
Osmotic pressure is the pressure thats in general needed to stop Osmosis. Or, in other words, to stop the water from getting diffused from the membrane. Osmotic pressure gets determined by how to concentrate the solute is.
By osmotic pressure, diffusion happens from high to low concentration areas.
Importance of Osmosis
Osmosis is a process that is important for both plants and animals. You can say that this process happens in both plants and animals. We have listed below the importance of Osmosis in Plants and Animals in different ways.
Importance of Osmosis in Plants
- The water levels in a plant cell remain maintained through Osmosis.
- The softer cells of a plant body get turgidity.
- The conduction of water gets controlled from xylem elements to the adjacent cells.
- The more the osmotic pressure, the more the resistance to drought injury in plants.
Importance of Osmosis in Animals
- The flow of dissolved solids, liquids, and gases cells gets regulated by Osmosis.
- Osmosis also helps with the absorption of water from the intestines to the blood.
- The selective allowance of substances in and out of the cell helps in the release of toxic waste.
Example of Osmosis
Below are the examples of Osmosis:
- When plant roots absorb the water from the soil.
- Being in the water for a longer period causes the skin of the hands to go prune. The reason is that skin cells absorb the water, and the skin expands.
- Suppose a freshwater fish gets placed in water having different salt concentrations. The result is that the fish dies. That is because of the entry and exit of water in fish cells.
Final Thoughts
Osmosis is an important process for all living beings. But sometimes, Osmosis can also have adverse effects.
So that is all about the osmosis process and its types. That’s all for now. Thanks for reading.